Ионные каналы
Изучение структур ионных каналов имеет ключевое значение для понимания многих физиологических процессов и патофизиологических состояний. Они играют критическую роль в регуляции клеточной возбудимости, передачи сигналов и поддержании гомеостаза. Понимание структуры каналов позволяет раскрыть механизмы работы этих молекулярных комплексов и взаимодействия с лекарственными средствами. Знания в данной области выступают в качестве фундамента для разработки новых терапевтических подходов для лечения тяжелых заболеваний, связанных с нарушениями в работе ионных каналов — эпилепсия, сердечно-сосудистые болезни и нейродегенеративные расстройства.
Публикации группы по направлению
A Deep Learning Approach to Uncover Voltage-Gated Ion Channels’ Intermediate States
Julia Kacher, Olga S. Sokolova, and Mounir Tarek
Owing to recent advancements in cryo-electron microscopy, voltage-gated ion channels have gained a greater comprehension of their structural characteristics. However, a significant enigma remains unsolved for a large majority of these channels: their gating mechanism. This mechanism, which encompasses the conformational changes between open and closed states, is pivotal to their proper functioning. Beyond the binary states of open and closed, an ensemble of intermediate states defines the transition path in-between. Due to the lack of experimental data, one might resort to molecular dynamics simulations as an alternative to decipher these states and the transitions between them. However, the high-energy barriers and the colossal time scales involved hinder access to the latter. We present here an application of deep learning as a reliable pipeline for a comprehensive exploration of voltage-gated ion channel conformational rearrangements during gating. We showcase the pipeline performance specifically on the Kv1.2 voltage sensor domain and confront the results with existing data. We demonstrate how our physics-based deep learning approach contributes to the theoretical understanding of these channels and how it might provide further insights into the exploration of channelopathies. DOI
Variable Clinical Appearance of the Kir2.1 Rare Variants in Russian Patients with Long QT Syndrome
Elena Zaklyazminskaya, Margarita Polyak, Anna Shestak, Mariam Sadekova, Vera Komoliatova, Irina Kiseleva, Leonid Makarov, Dmitriy Podolyak, Grigory Glukhov, Han Zhang, Denis Abramochkin, Olga S Sokolova
Background: The KCNJ2 gene encodes inward rectifier Kir2.1 channels, maintaining resting potential and cell excitability. Presumably, clinical phenotypes of mutation carriers correlate with ion permeability defects. Loss-of-function mutations lead to QTc prolongation with variable dysmorphic features, whereas gain-of-function mutations cause short QT syndrome and/or atrial fibrillation.
Methods: We screened 210 probands with Long QT syndrome for mutations in the KCNJ2 gene. The electrophysiological study was performed for the p.Val93Ile variant in the transfected CHO-K1 cells.
Results: We found three rare genetic variants, p.Arg67Trp, p.Val93Ile, and p.R218Q, in three unrelated LQTS probands. Probands with p.Arg67Trp and p.R218Q had a phenotype typical for Andersen-Tawil (ATS), and the p.Val93Ile carrier had lone QTc prolongation. Variant p.Val93Ile was initially described as a gain-of-function pathogenic mutation causing familial atrial fibrillation. We validated electrophysiological features of this variant in CHO-K1 cells, but no family members of these patients had atrial fibrillation. Using ACMG (2015) criteria, we re-assessed this variant as a variant of unknown significance (class III).
Conclusions: LQT7 is a rare form of LQTS in Russia, and accounts for 1% of the LQTS cohort. Variant p.Val93Ile leads to a gain-of-function effect in the different cell lines, but its clinical appearance is not so consistent. The clinical significance of this variant might be overestimated. DOI

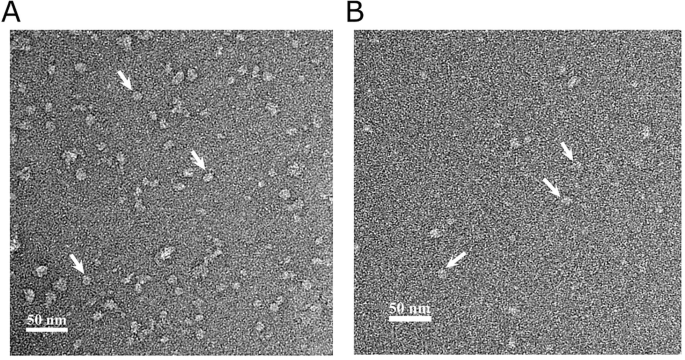
Purification of Potassium Ion Channels Using Styrene–Maleic Acid Copolymers
Glukhov Grigory, Karlova Maria, Kravchuk Ekaterina, Glukhova Anna, Trifonova Elizaveta, Sokolova Olga S.
Structural studies require the production of target proteins in large quantities and with a high degree of purity. For membrane proteins, the bottleneck in determining their structure is the extraction of the target protein from the cell membranes. A detergent that improperly mimics the hydrophobic environment of the protein of interest can also significantly alter its structure. Recently, using lipodiscs with styrene–maleic acid (SMA), copolymers became a promising strategy for the purification of membrane proteins. Here, we describe in detail the one-step affinity purification of potassium ion channels solubilized in SMA and sample preparation for future structural studies. DOI