VIruses
The scientific activity of the Structural Biotechnology group in the field of virology is focused on both bacteriophages and eukaryotic viruses. The study of virions using Cryo-EM is a strong complement to modern methods of genome analysis. Data on the geometry and protein composition are necessary both for the possible biotechnological application of viruses and for the potential treatment of currently incurable diseases.
Selected publications on the topic
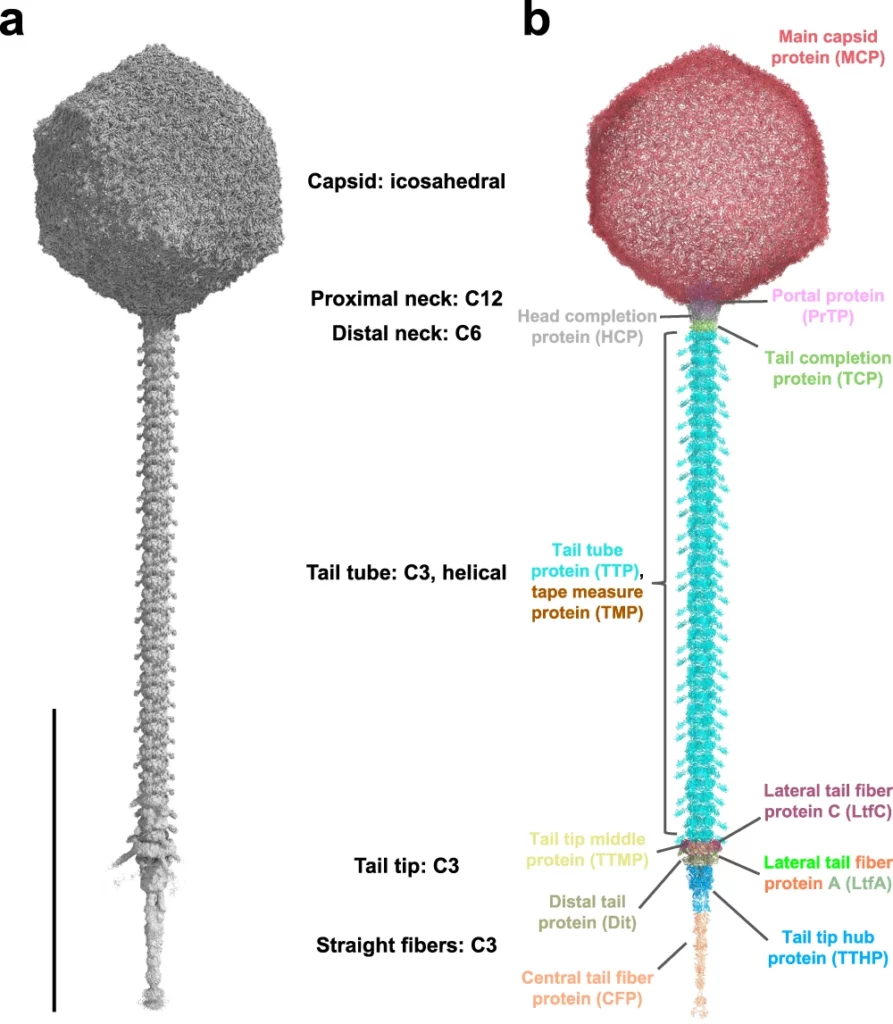
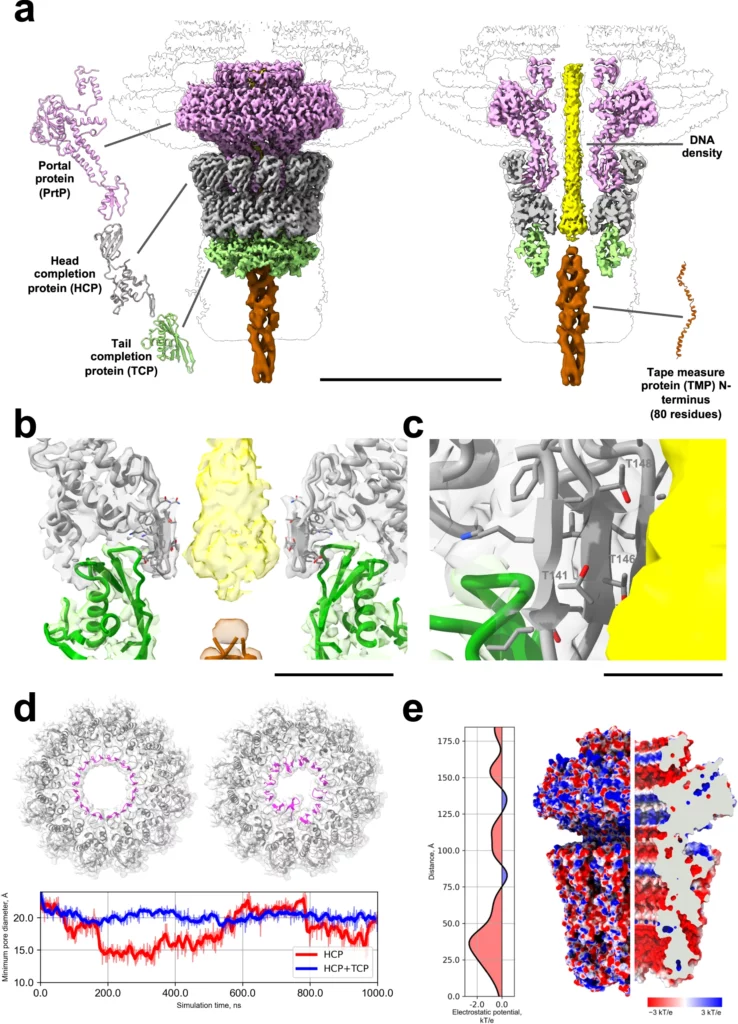
Nearly complete structure of bacteriophage DT57C reveals architecture of head-to-tail interface and lateral tail fibers.
Ayala, R., Moiseenko, A.V., Chen, T.H., Kulikov, E.E., Golomidova, A.K., Orekhov, P.S., Street, M.A., Sokolova, O.S., Letarov, A.V., Wolf, M.
The T5 family of viruses are tailed bacteriophages characterized by a long non-contractile tail. The bacteriophage DT57C is closely related to the paradigmal T5 phage, though it recognizes a different receptor (BtuB) and features highly divergent lateral tail fibers (LTF). Considerable portions of T5-like phages remain structurally uncharacterized. Here, we present the structure of DT57C determined by cryo-EM, and an atomic model of the virus, which was further explored using all-atom molecular dynamics simulations. The structure revealed a unique way of LTF attachment assisted by a dodecameric collar protein LtfC, and an unusual composition of the phage neck constructed of three protein rings. The tape measure protein (TMP) is organized within the tail tube in a three-stranded parallel α-helical coiled coil which makes direct contact with the genomic DNA. The presence of the C-terminal fragment of the TMP that remains within the tail tip suggests that the tail tip complex returns to its original state after DNA ejection. Our results provide a complete atomic structure of a T5-like phage, provide insights into the process of DNA ejection as well as a structural basis for the design of engineered phages and future mechanistic studies. DOI
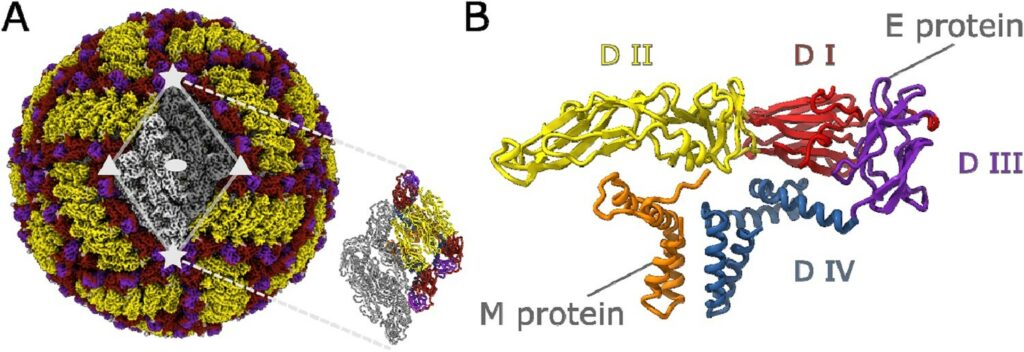
Structural diversity of tick-borne encephalitis virus particles in the inactivated vaccine based on strain Sofjin
Andrey Moiseenko, Yichen Zhang, Mikhail F. Vorovitch, Alla L. Ivanova, Zheng Liu, Dmitry I. Osolodkin, Alexey M. Egorov, Aydar A. Ishmukhametov, Olga S. Sokolova
The main approach to preventing tick-borne encephalitis (TBE) is vaccination. Formaldehyde-inactivated TBE vaccines have a proven record of safety and efficiency but have never been characterized structurally with atomic resolution. We report a cryoelectron microscopy (cryo-EM) structure of the formaldehyde-inactivated TBE virus (TBEV) of Sofjin-Chumakov strain representing the Far-Eastern subtype. A 3.8 Å resolution reconstruction reveals the structural integrity of the envelope E proteins, specifically the E protein ectodomains. The comparative study shows a high structural similarity to the previously published structures of the TBEV European subtype strains Hypr and Kuutsalo-14. A fraction of inactivated virions exhibits asymmetric features including the deformations of the membrane profile. We propose that the heterogeneity is caused by inactivation and perform a local variability analysis on the small parts of the envelope protein shell to reveal membrane curvature features possibly induced by the inactivation. The results of this study will have implications for the design of novel vaccines against diseases caused by flaviviruses. DOI